Recombinant human PD-L1 protein (Active) (ab167713)
Key features and details
- Expression system: HEK 293 cells
- Purity: > 98% SDS-PAGE
- Endotoxin level:
- Active: Yes
- Tags: His tag C-Terminus
- Suitable for: HPLC, SDS-PAGE, Functional Studies
Preparation and Storage
-
Applications
HPLC
SDS-PAGE
Functional Studies
-
Form
Lyophilized -
Additional notes
For a recombinant rabbit monoclonal antiody to PD-L1 in IHC usage and KO validated - see ab205921 (clone 28-8)
For a recombinant rabbit monoclonal antiody to PD-L1 in WB and ICC/IF usage - see ab174838 (clone EPR1161(2))
-
 Concentration information loading...
Concentration information loading...
Images
-
Immobilized ab167713 at 1 μg/mL (100 μL/well) binds Anti-Human PD-L1 MAb (Human IgG1).
Linear range of 0.1-3 ng/mL (QC tested).
-
The purity of Human PD-L1 (His Tag) (HPLC verified) was greater than 90% as determined by SEC-HPLC.
-
Immobilized ab167713 on Anti-His Tag (C-term) Antibody, precoated (0.1 μg/well) plate, binds Human PD-1, Mouse IgG2a Fc Tag (HPLC-verified), at 5 μg/mL (100 μL/well).
Linear range of 10-78 ng/mL (Routinely tested).
-
Loaded Recombinant human PD1 protein (Fc Chimera Active) ab221398 on ProteinA Biosensor, can bind Human PD-L1, His Tag ab167713 with an affinity constant of 5.3 μM as determined in BLI assay.
-
Biotinylated Human PD-1, Fc Tag (ab246137) binds Recombinant human PD-L1 protein ab167713 at 1 μg/mL (5μL/well).
Linear range of 0.02-0.625 μg/mL as determined in a alphaLISA homogeneous assay (Routinely tested).
-
Human PD-1, Fc Tag (ab221398), captured on CM5 chip via anti-human IgG Fc antibody, binds ab167713 with an affinity constant of 3.6 μM, as determined in an SPR assay (Biacore T200).
-
Anti-Human PD-L1 Mab (Human IgG1), captured on CM5 chip via anti-human IgG Fc antibodies surface, binds ab167713 with an affinity constant of 0.286 nM, as determined in a SPR assay (Biacore T200) (Routinely tested).
-
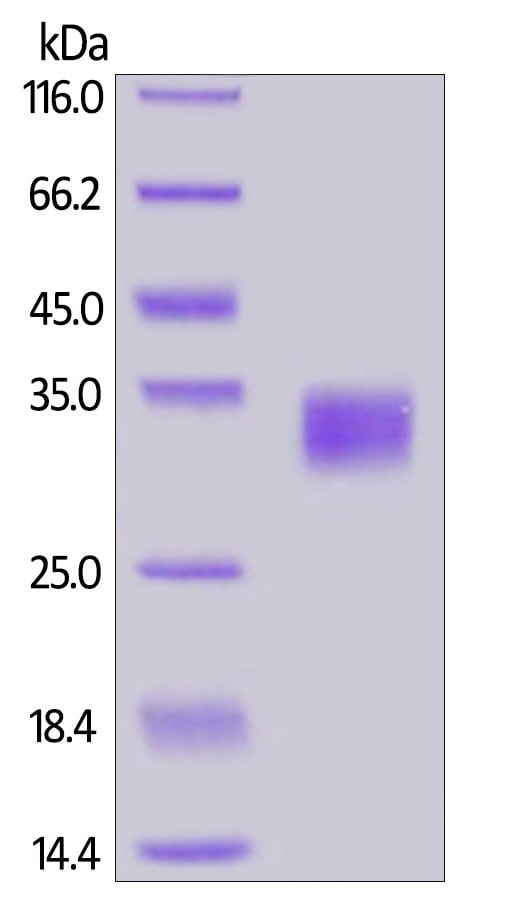
Reduced ab167713 on SDS-PAGE, stained overnight with Coomassie Blue.
The purity of the protein is greater than 98%.
The protein migrates as 30-35 kDa under reducing conditions, due to glycosylation.