Recombinant Serine/threonine-protein phosphatase (ab188459)
Key features and details
- Expression system: Escherichia coli
- Purity: > 95% SDS-PAGE
- Active: Yes
- Suitable for: SDS-PAGE, Functional Studies
-
Product name
Recombinant Serine/threonine-protein phosphatase -
Biological activity
400,000 U/mg. 400 U/µl, where one unit is defined as the amount of enzyme that hydrolyzes 1 nmole of p-nitrophenyl phosphate per minute at 30°C. Unit definition assays are performed with 50mM p-nitrophenyl phosphate in λ-PPase buffer, supplemented with 2 mM MnCl2 in a 50 µl reaction.
-
Purity
> 95 % SDS-PAGE. -
Expression system
Escherichia coli -
Accession
-
Protein length
Full length protein -
Animal free
No -
Nature
Recombinant -
-
Species
Bacteriophage lambda -
Sequence
MRYYEKIDGSKYRNIWVVGDLHGCYTNLMNKLDTIGFDNKKDLLISVGDL VDRGAENVECLELITFPWFRAVRGNHEQMMIDGLSERGNVNHWLLNGGGW FFNLDYDKEILAKALAHKADELPLIIELVSKDKKYVICHADYPFDEYEFG KPVDHQQVIWNRERISNSQNGIVKEIKGADTFIFGHTPAVKPLKFANQMY IDTGAVFCGNLTLIQVQGEGA -
Predicted molecular weight
25 kDa -
Amino acids
1 to 221 -
Additional sequence information
From Bacteriophage lambda
-
Images
-
NPM1 protein in HeLa cells treated with Nocodazole was lyzed, and immunoprecipited with ab188459. The precipitate was suspended in 50 μl of
λ protein phosphatase reaction buffer, with 5 μl of the protein phosphatase and incubated at 30℃. The reaction products were analyzed by western blotting.
-
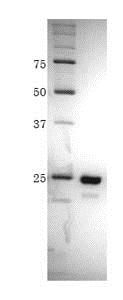
SDS-PAGE analysis of ab188459.