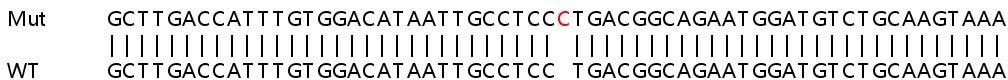Human EXOC2 knockout HeLa cell line (ab265839)
Overview
-
Product name
Human EXOC2 knockout HeLa cell line -
Parental Cell Line
HeLa -
Organism
Human -
Mutation description
Knockout achieved by using CRISPR/Cas9, 1 bp insertion in exon 3 and 2 bp insertion in exon 3 -
Passage number
Knockout validation
Sanger SequencingTested applications
Suitable for: WBmore detailsBiosafety level
2General notes
Western blot data indicates that the CRISPR gene edit may have resulted in a truncation of the protein of interest. Please see data images.
Recommended control: Human wild-type HeLa cell line (ab255928). Please note a wild-type cell line is not automatically included with a knockout cell line order, if required please add recommended wild-type cell line at no additional cost using the code WILDTYPE-TMTK1.
Cryopreservation cell medium: Cell Freezing Medium-DMSO Serum free media, contains 8.7% DMSO in MEM supplemented with methyl cellulose.
Culture medium: DMEM (High Glucose) + 10% FBS
Initial handling guidelines: Upon arrival, the vial should be stored in liquid nitrogen vapor phase and not at -80ºC. Storage at -80ºC may result in loss of viability.
1. Thaw the vial in 37ºC water bath approximately 1-2 minutes.
2. Transfer the cell suspension (0.8 ml) to a 15 ml/50 ml conical sterile polypropylene centrifuge tube containing 8.4 ml pre-warmed culture medium, wash vial with an additional 0.8 ml culture medium (total volume 10 ml) to collect remaining cells, and centrifuge at 201 x g (rcf) for 5 minutes at room temperature. 10 ml represents minimum recommended dilution. 20 ml represents maximum recommended dilution.
3. Resuspend the cell pellet in 5 ml pre-warmed culture medium and count using a haemocytometer (Click here to view haemocytometer protocol) or alternative cell counting method. Based on cell count, seed cells in an appropriate cell culture flask at a density of 2x104 cells/cm2. This should allow for confluency within 48 hours. Seeding density is given as a guide only and should be scaled to align with individual lab schedules.
4. Incubate the culture at 37ºC incubator with 5% CO2. Cultures should be monitored daily.Subculture guidelines:
- All seeding densities should be based on cell counts gained by established methods.
- A guide seeding density of 2x104 cells/cm2 is recommended for confluency (80-90% confluence) within 48 hours.
- A partial media change 24 hours prior to subculture may be helpful to encourage growth, if required.
- Cells should be passaged when they have achieved 80-90% confluence.
Click here to view the Mammalian cell tissue culture protocol
This product is subject to limited use licenses from The Broad Institute, ERS Genomics Limited and Sigma-Aldrich Co. LLC, and is developed with patented technology. For full details of the licenses and patents please refer to our limited use license and patent pages.
Properties
-
Number of cells
1 x 106 cells/vial, 1 mL -
Viability
~90% -
Adherent /Suspension
Adherent -
Tissue
Cervix -
Cell type
epithelial -
Disease
Adenocarcinoma -
Gender
Female -
STR Analysis
Amelogenin X D5S818: 11, 12 D13S317: 12, 13.3 D7S820: 8, 12 D16S539: 9, 10 vWA: 16, 18 TH01: 7 TPOX: 8,12 CSF1PO: 9, 10 -
Mycoplasma free
Yes -
Storage instructions
Shipped on Dry Ice. Store in liquid nitrogen. -
Storage buffer
Constituents: 8.7% Dimethylsulfoxide, 2% Cellulose, methyl ether -
Research areas
Target
-
Function
Component of the exocyst complex involved in the docking of exocytic vesicles with fusion sites on the plasma membrane. -
Tissue specificity
Widely expressed with highest levels in brain and placenta. -
Sequence similarities
Belongs to the SEC5 family.
Contains 1 IPT/TIG domain. -
Domain
Interacts with RALA through the TIG domain. - Information by UniProt
Properties
-
Number of cells
1 x 106 cells/vial, 1 mL -
Viability
~90% -
Adherent /Suspension
Adherent -
Tissue
Cervix -
Cell type
epithelial -
Disease
Adenocarcinoma -
Gender
Female -
STR Analysis
Amelogenin X D5S818: 11, 12 D13S317: 12, 13.3 D7S820: 8, 12 D16S539: 9, 10 vWA: 16, 18 TH01: 7 TPOX: 8,12 CSF1PO: 9, 10 -
Mycoplasma free
Yes -
Storage instructions
Shipped on Dry Ice. Store in liquid nitrogen. -
Storage buffer
Constituents: 8.7% Dimethylsulfoxide, 2% Cellulose, methyl ether -
Research areas
Images
-
Allele-1: 1 bp insertion in exon 3.
-
All lanes : Anti-EXOC2 antibody [EPR9420] (ab140620) at 1000 µg
Lane 1 : Wild-type HeLa cell lysate
Lane 2 : EXOC2 knockout HeLa cell lysate
Lane 3 : SH-SY5Y cell lysate
Lane 4 : Daudi cell lysate
Lysates/proteins at 20 µg per lane.
Performed under reducing conditions.
Predicted band size: 104 kDa
Observed band size: 104 kDaLanes 1- 4: Merged signal (red and green). Green - ab140620 observed at 104 kDa. Red - Anti-GAPDH antibody [6C5] - Loading Control (ab8245) observed at 37 kDa.
ab140620 was shown to react with EXOC2 in wild-type HeLa cells in western blot. The band observed in knockout cell line ab265839 (knockout cell lysate ab257944) lane below 104kDa may represent truncated forms and cleaved fragments. This has not been investigated further. Wild-type HeLa and EXOC2 knockout HeLa cell lysates were subjected to SDS-PAGE. Membrane was blocked for 1 hour at room temperature in 0.1% TBST with 3% non-fat dried milk. ab140620 and Anti-GAPDH antibody [6C5] - Loading Control (ab8245) were incubated overnight at 4°C at a 1 in 1000 dilution and a 1 in 20000 dilution respectively. Blots were developed with Goat anti-Rabbit IgG H&L (IRDye®800CW) preadsorbed (ab216773) and Goat anti-Mouse IgG H&L (IRDye®680RD) preadsorbed (ab216776) secondary antibodies at 1 in 20000 dilution for 1 hour at room temperature before imaging.
-
Allele-2: 2 bp insertion in exon 3.