Glycine Assay Kit (Fluorometric) (ab211100)
Key features and details
- Assay type: Quantitative
- Detection method: Fluorescent
- Platform: Microplate reader
- Sample type: Cell Lysate, Plasma, Saliva, Serum, Tissue Lysate, Urine
- Sensitivity: 1 µM
Overview
-
Product name
Glycine Assay Kit (Fluorometric) -
Detection method
Fluorescent -
Sample type
Saliva, Urine, Serum, Plasma, Cell Lysate, Tissue Lysate -
Assay type
Quantitative -
Sensitivity
1 µM -
Species reactivity
Reacts with: Mammals, Other species -
Product overview
Glycine Assay Kit (Fluorometric) (ab211100) provides a provides a simple, sensitive, and high-throughput adaptable assay that detects physiological concentrations of glycine (GLY) in multiple biological samples such as cell and tissue lysates and biological fluids. In this assay, Glycine is oxidized in the presence of GLY Enzyme Mix is converted to an intermediate, which reacts with the fluorescent probe to generate a strong stable signal at Ex/Em = 535/587 nm. The intensity of the signal is directly proportional to the amount of GLY in the sample.
The reaction is specific and other amino acids do not interfere with the assay. The assay can detect as little as 1 µM of Glycine in a variety of biological samples.
-
Notes
Glycine (GLY, G) is one of the 20 standard amino acids commonly found in proteins. Glycine’s side chain is a hydrogen substituent, which makes it the smallest proteogenic and the only non-chiral amino acid. Basic functions of Glycine include the participation in the synthesis of creatine, glutathione, heme groups, and conjugated bile acids (bile salts). It is also present as one of the most abundant residues in the triple-helical structure of collagen.
Glycine acts as a glucogenic amino acid by regulating sugar levels in blood. Therefore, glycine supplementation has been used in patients suffering anemia, hypoglycemia and chronic fatigue. Glycine possesses both inhibitory and excitatory neurotransmitter functions in the brain stem and spinal cord. In cancer cells, glycine consumption is highly correlated to cancer cell proliferation via purine synthesis. Glycine uptake in cancer cell studies supports the role of this amino acid in tumorigenesis and malignancy.
-
Platform
Microplate reader
Properties
-
Storage instructions
Store at -20°C. Please refer to protocols. -
Components 100 tests GLY Assay Buffer 1 x 25ml GLY Developer (200 µg) 1 vial GLY Enzyme Mix (600 mU) 1 vial GLY Probe 1 x 400µl GLY Standard (10 µM) 1 vial -
Research areas
-
Relevance
Defects in GLDC are a cause of nonketotic hyperglycinemia (NKH), also known as glycine encephalopathy (GCE). NKH is an autosomal recessive disease characterized by accumulation of a large amount of glycine in body fluid and by severe neurological symptoms. The degredation of glycine is catalised by the glycine cleavage system. The P protein binds the alpha-amino group of glycine through its pyridoxal phosphate cofactor; carbondioxide is released and the remaining methylamine moiety is then transferred to the lipoamide cofactor of the H protein. The glycine cleavage system is composed of four proteins: P, T, L and H.
Images
-
Typical Glycine standard calibration curve.
-
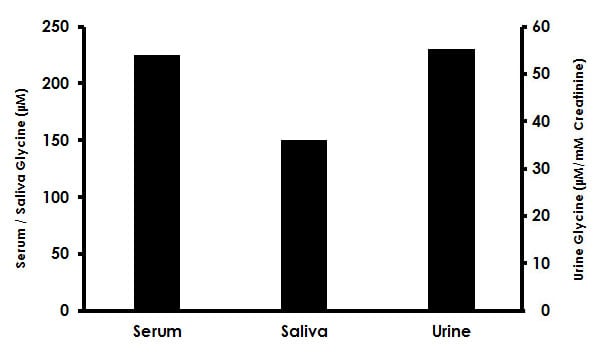
Estimation of Glycine in human serum, saliva, and urine. Samples were deproteinized using 10 kD spin column (ab93349) and diluted with GLY Assay Buffer prior to performing assay: (serum 1:64 dilution; saliva 1:32 dilution; urine 1:128 dilution). 25 µL of each diluted sample was spiked with 0.3 nmol of Glycine Standard and assayed following the kit protocol. Glycine concentrations in serum (224 ± 21µM), saliva (149 ± 7 µM) and urine (54 ± 4 µM/mM creatinine).