Vedolizumab ELISA Kit (Entyvio®) (ab264503)
Key features and details
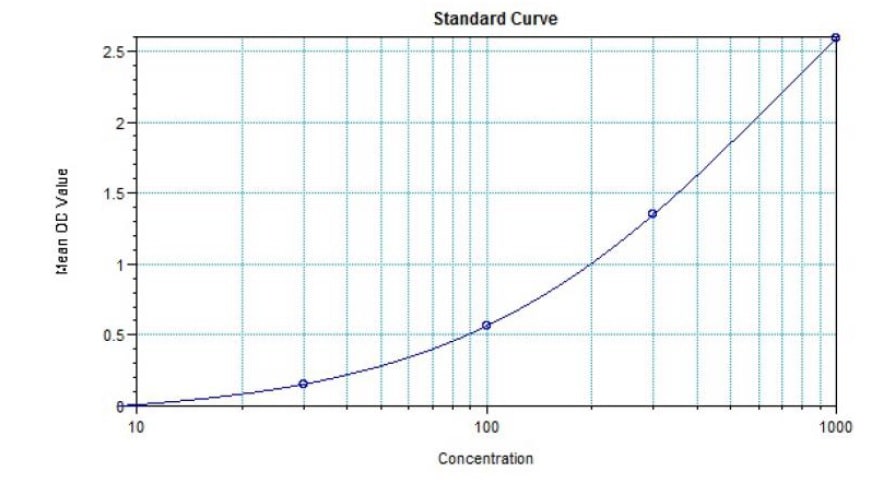
- Sensitivity: 30 ng/ml
- Range: 30 ng/ml - 1000 ng/ml
- Sample type: Plasma, Serum
- Detection method: Colorimetric
- Assay type: Quantitative
- Reacts with: Human
Overview
-
Product name
Vedolizumab ELISA Kit (Entyvio®) -
Detection method
Colorimetric -
Precision
Intra-assay Sample n Mean SD CV% Overall Inter-assay Sample n Mean SD CV% Overall -
Sample type
Serum, Plasma -
Assay type
Quantitative -
Sensitivity
30 ng/ml -
Range
30 ng/ml - 1000 ng/ml -
Recovery
Sample specific recovery Sample type Average % Range Serum 85% - 115% -
Assay duration
Multiple steps standard assay -
Species reactivity
Reacts with: Human -
Product overview
Vedolizumab ELISA Kit (Entyvio®) (ab264503) is a Solid phase enzyme-linked immunosorbent assay (ELISA) based on the sandwich principle. The color developed is proportional to the amount of Vedolizumab in the sample or standard.
Vedolizumab is a recombinant humanized IgG1 monoclonal antibody directed against the human lymphocyte α4β7 integrin, a key mediator of gastrointestinal inflammation. It is used in the treatment of moderate to severe active ulcerative colitis and Crohn's disease for patients who have had an inadequate response with, lost response to, or were intolerant to inhibitors of tumor necrosis factor-alpha (TNF-alpha) or other conventional therapies.
-
Tested applications
Suitable for: Sandwich ELISAmore details -
Platform
Microplate
Properties
-
Storage instructions
Store at +4°C. Please refer to protocols. -
Components 1 x 96 tests Assay Buffer 2 x 50ml HRP-conjugate Probe 1 x 12ml Micro ELISA Plate 1 unit Plate Sealers 2 units Stop Solution 1 x 12ml TMB Substrate 1 x 12ml Vedolizumab Standard S1 1 x 300µl Vedolizumab Standard S2 1 x 300µl Vedolizumab Standard S3 1 x 300µl Vedolizumab Standard S4 1 x 300µl Vedolizumab Standard S5 1 x 300µl Vedolizumab Standard S6 1 x 300µl Vedolizumab Standard S7 1 x 300µl Wash Buffer (20X) 1 x 50ml