MitoBiogenesis™ In-Cell ELISA Kit (Colorimetric) (ab110217)
Key features and details
- Assay type: Cell-based (quantitative)
- Detection method: Colorimetric
- Sample type: Adherent cells
- Reacts with: Mouse, Rat, Cow, Human
Overview
-
Product name
MitoBiogenesis™ In-Cell ELISA Kit (Colorimetric)
See all MitoBiogenesis kits -
Detection method
Colorimetric -
Sample type
Adherent cells -
Assay type
Cell-based (quantitative) -
Assay duration
Multiple steps standard assay -
Species reactivity
Reacts with: Mouse, Rat, Cow, Human -
Product overview
For identifying inhibitors and activators of mitochondrial biogenesis in adherent cultured cells. Each kit contains sufficient reagents to analyze two 96-well plates of fixed human, rat, mouse, or bovine cells. This kit utilizes colorimetric detection for use with standard plate readers. An alternate IR version of this kit is available which utilizes LI-COR® near-infrared IRDyes® for detection - MitoBiogenesis™ In-Cell ELISA Kit (IR) (ab110216/MS642).
In-Cell ELISA Kits use quantitative immunocytochemistry to measure protein levels or post-translational modifications in cultured cells. Cells are fixed in a 96-well plate and targets of interest are detected with highly specific, well-characterized monoclonal antibodies and levels are quantified with enzyme-labeled secondary antibodies.
ab110217 MitoBiogenesis™ In-Cell ELISA Kit (ab110217/MS643) is designed to measure drug-induced effects on mitochondrial biogenesis early in the safety screening process. ab110217 MitoBiogenesis™ In-Cell ELISA Kit is a true duplexing 96/384-well assay that ratios both an mtDNA- and an nDNA-encoded protein in cultured or primary cells, and which requires very little sample prep and few overall steps.
Cells (human, rat or mouse) are seeded in 96- or 384-well microplates, and after exposure to experimental compounds for several cell doublings, the levels of two mitochondrial proteins are measured simultaneously in each well. The two proteins are each subunits of a different oxidative phosphorylation enzyme complex, one protein being subunit I of Complex IV (COX-I), which is mtDNA-encoded, and the other being the 70kDa subunit of Complex II (SDH-A), which is nDNA-encoded. Complex IV includes several proteins which are encoded in the mitochondrion, while the proteins of Complex II are entirely encoded in the nucleus. Optionally, total protein levels can also be measured.
LI-COR®, Odyssey®, Aerius® and IRDye® are registered trademarks of LI-COR Biosciences Inc
Plates are available in our ICE (In-Cell ELISA) Support Pack (ab111542) which can be bought separately.
-
Notes
Related products
Review the mitochondrial assay guide, or the full metabolism assay guide to learn about more assays for metabolites, metabolic enzymes, mitochondrial function, and oxidative stress, and also how to assay metabolic function in live cells using your plate reader.
Abcam has not and does not intend to apply for the REACH Authorisation of customers’ uses of products that contain European Authorisation list (Annex XIV) substances.
It is the responsibility of our customers to check the necessity of application of REACH Authorisation, and any other relevant authorisations, for their intended uses. -
Platform
Microplate
Properties
-
Storage instructions
Store at +4°C. Please refer to protocols. -
Components 2 x 96 tests 100X Triton X-100 1 x 1.5ml 10X Blocking Buffer 1 x 15ml 10X Phosphate Buffered Saline 1 x 100ml 1X AP Development Solution 1 x 24ml 1X HRP Development Solution 1 x 24ml 200X Primary Antibodies 1 x 0.1ml 2500X AP-labeled Secondary Antibody 1 x 12µl 2500X HRP-labeled Secondary Antibody 1 x 12µl 400X Tween-20 1 x 2ml AP Development Reagent 1 x 139mg 1X Janus Green Stain 1 x 17ml Plate Seals 2 units -
Research areas
-
Alternative names
- MS641
- MS642
- MS643
- MS644
Images
-
Antibody specificity demonstrated by Western Blot. A Western blot of total cell protein (10 µg) from human or rat cultured cells was probed with the primary and secondary antibodies and scanned with a LI-COR® Odyssey® imager. The two mitochondrial proteins targeted by the two primary mAbs were labeled and visualized specifically despite the presence of thousands of other proteins. Furthermore, reduction of mtDNA levels in human Rho0 (mtDNA-depleted) cells, or inhibition of mitochondrial protein translation by chloramphenicol in rat cells both result in specific reduction of COX-I protein while nuclear DNA-encoded SDH-A is unaffected.
-
Quantitative measurement of the COX-I/SDH-A protein expression ratio. At all cell concentrations, a consistent ratio of mtDNA-encoded protein expression (COX-I) to nuclear DNA-encoded mitochondrial protein expression (SDH-A) is observed in untreated cells. Therefore, normalizing COX-I levels to SDH-A levels simplifies data analysis and eliminates the need to perform all tests at the same cell concentration.
-
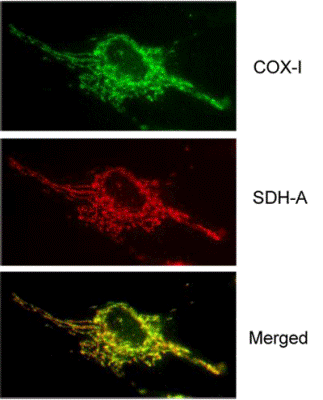
Antibody specificity demonstrated by immunocytochemistry. Two-color immunocytochemical labeling of cultured cells with the two MS643 primary monoclonal antibodies specific for COX-I and SDH-A. The two antibodies exhibit striking and specific co-localization in the mitochondria, consistent with the known mitochondrial expression of both proteins.
-
Inhibition of mitochondrial biogenesis by chloramphenicol The IC50 of a drug's effect on mitochondrial protein translation can be determined quickly using the MitoBiogenesis™ In-Cell ELISA Kit. In this example, cells were seeded at 6000 cells/well, allowed to grow for 3 cell doublings in a drug dilution series and then the relative amounts of COX-I, and SDH-A were measured in each well. Chloramphenicol inhibits mtDNA-encoded COX-I protein synthesis relative to nuclear DNA-encoded SDH-A protein synthesis by 50% at 8.1 µM.
-
Cells are grown to ~80% confluency in a 96- or 384-well plate, a drug/other treatment is applied to stimulate a cellular response. The cells are then fixed and permeabilized, effectively "freezing" them. Primary antibodies are then added which bind to their intended targets within the mitochondria or other subcellular compartment. After incubation, the unbound primary antibodies are washed away and secondary antibodies are added. These secondaries are conjugated to either IRDyes® or to an enzyme label (HRP or AP) for the colorimetric versions of the assays. Unbound secondaries are washed away, reaction buffer is added for the colorimetric assays, and the signal is read on a suitable instrument for the kit type.
» In-cell ELISA diagram in PDF format