ioGlutamatergic Neurons – Human iPSC-Derived Glutamatergic Neurons (ab259259)
Overview
-
Product name
ioGlutamatergic Neurons – Human iPSC-Derived Glutamatergic Neurons -
Description
ioGlutamatergic Neurons - Human iPSC-Derived Glutamatergic Neurons -
Parental Cell Line
iPSC -
Organism
Human -
Tested applications
Suitable for: High throughput screening, Functional Studies, ICC/IF, CellDiffmore details -
Biosafety level
1 -
General notes
For non-profit organizations, please use discount code ACCESS-TMA72 for a discounted price.
Product is available in three sizes:
Small - 7.5x105 cells/vial
Large - 1.5x106 cells/vial
Multipack - 4 x Large vials - total 6x106 cellsioGlutamatergic Neurons have been reprogrammed from human induced pluripotent stem cells (iPSC) using opti-ox, a precise reprogramming technology. Human stem cells, within days, convert into consistent, mature, functional glutamatergic neurons providing a high quality human model for the study of neurological activity and disease.
ioGlutamatergic Neurons cultures consist mainly of glutamatergic neurons (>80%) characterised by the expression of the glutamate transporter genes VGLUT1 and VGLUT2 (see Figure 1). The minor remaining fraction of the neuronal population express marker genes of cholinergic neurons. A bulk RNA-seq analysis shows that ioGlutamatergic Neurons have a rostral CNS identity and express the classical cortical maker genes FOXG1 and TBR1 (data not shown).
In partnership with bit.bio
Karyotype: Normal
Seeding Density: 30,000 cells/cm2
Seeding compatibility: 6-, 12-, 24-, 96- and 384-well compatible
Quality control: ICC and gene expression analysis
Research applications: Academic research, Drug development, Neurotoxicology, Genetic screening (e.g. CRISPR screening).
This product is subject to limited use licenses from iPS Academia Japan Inc, TET Systems GmbH, ERS Genomics Limited and Sigma-Aldrich Co. LLC and is developed with Bit Bio patented technology. For full details of the licenses and patents please refer to our limited use license and patent pages.
Properties
-
Number of cells
Small - 7.5x105 cells/vial; Large - 1.5x106 cells/vial -
Viability
>85% -
Cell type
glutamatergic neuron -
Gender
Male -
Mycoplasma free
Yes -
Storage instructions
Shipped on Dry Ice. Store in liquid nitrogen. -
Storage buffer
Constituent: 10% DMSO
Images
-
ioGlutamatergic Neurons are compatible with plates ranging from 6 to 384 wells and are available in two vial sizes, tailored to suit your experimental needs with minimal waste.
- Recommended seeding density for ioGlutamatergic Neurons is 30,000 cells/cm2, compared to up to 250,000 cells/cm2 for other available products on the market.
- One Small vial (0.75 x 106 viable cells) can plate a minimum of 0.5 x 24-well plate, 0.75 x 96-well plate, or 1 x 384-well plate.
- One Large vial (1.5 x 106 viable cells) you can plate a minimum of 1 x 24-well plate, 1.5 x 96-well plate, or 2 x 384-well plates. -
 Immunocytochemistry/ Immunofluorescence - ioGlutamatergic Neurons – Human iPSC-Derived Glutamatergic Neurons (ab259259)
Immunocytochemistry/ Immunofluorescence - ioGlutamatergic Neurons – Human iPSC-Derived Glutamatergic Neurons (ab259259)Immunocytochemistry of ioGlutamatergic Neurons at day 9 post plating shows homogeneous expression of pan neuronal marker MAP2 and a dense neuronal network. Image courtesy of Charles River Laboratories.
-
 Immunocytochemistry/ Immunofluorescence - ioGlutamatergic Neurons – Human iPSC-Derived Glutamatergic Neurons (ab259259)
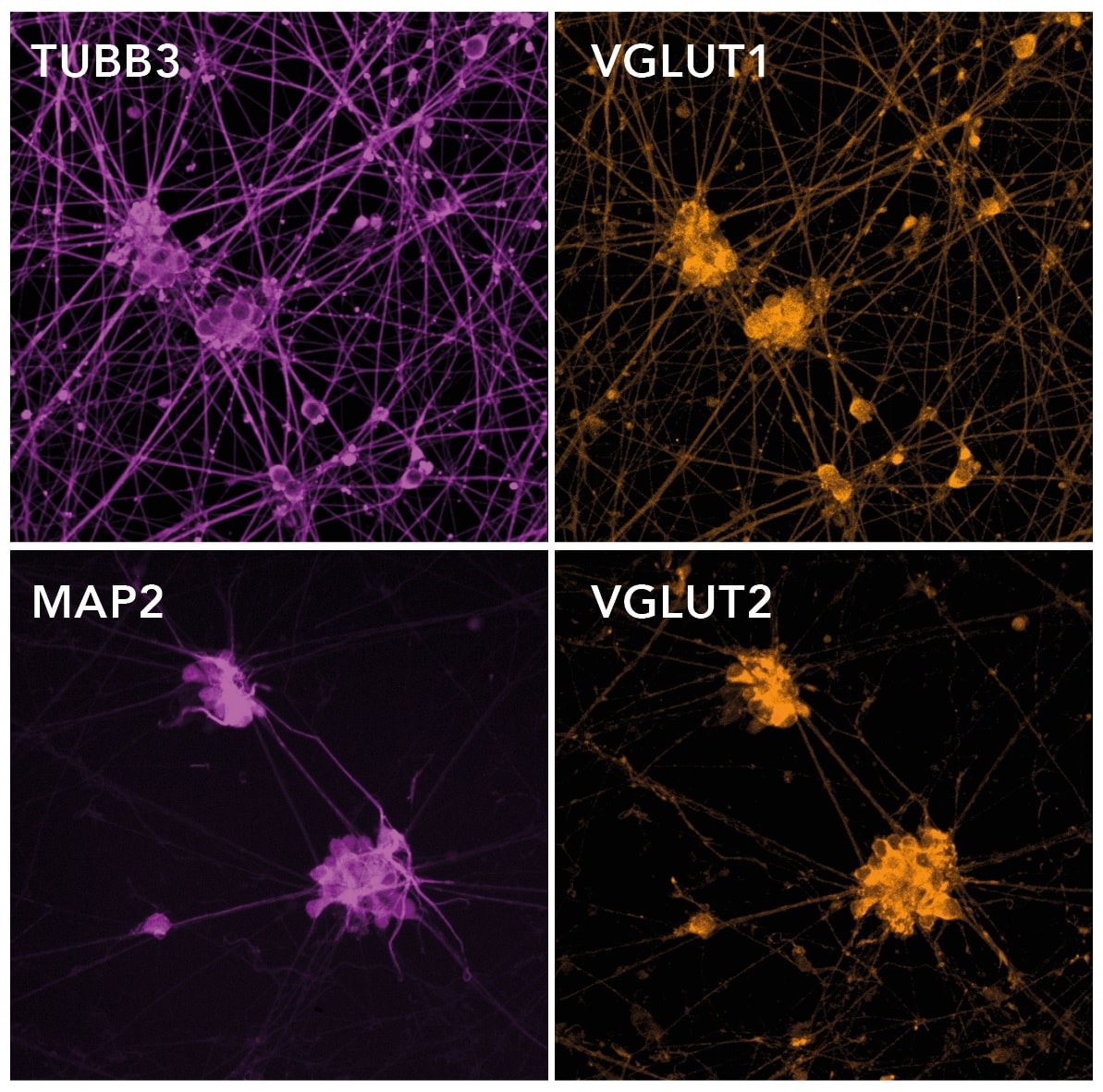
Immunocytochemistry/ Immunofluorescence - ioGlutamatergic Neurons – Human iPSC-Derived Glutamatergic Neurons (ab259259)Immunofluorescent staining on post-revival day 11 demonstrates homogenous expression of pan-neuronal proteins (MAP2 and TUBB3) and glutamatergic neuron-specific transporters (VGLUT1 and VGLUT2). Cells exhibit neurite outgrowth.
-
 Immunocytochemistry/ Immunofluorescence - ioGlutamatergic Neurons – Human iPSC-Derived Glutamatergic Neurons (ab259259)
Immunocytochemistry/ Immunofluorescence - ioGlutamatergic Neurons – Human iPSC-Derived Glutamatergic Neurons (ab259259)Immunofluorescence staining of NeuN using ab177487 in ioGlutamatergic Neurons (Human iPSC-Derived Glutamatergic Neurons, ab259259), which were differentiated for 1 day post induction.
The cells were fixed with 4% formaldehyde (10 min), permeabilized with 0.1% PBS-Tween for 5 mins and then blocked with 1% BSA/10% normal goat serum/0.3M glycine in 0.1% PBS-Tween for 1h. The cells were then incubated overnight at +4°C with ab177487 at 1 μg/ml and ab7291, Mouse monoclonal [DM1A] to alpha Tubulin, at 1/1000 dilution. Cells were then incubated with ab150081, Goat Anti-Rabbit IgG H&L (Alexa Fluor® 488) preadsorbed at 1/1000 dilution (shown in green) and ab150120, Goat Anti-Mouse IgG H&L (Alexa Fluor® 594) preadsorbed at 1/1000 dilution (shown in red). Nuclear DNA was labelled with DAPI (shown in blue).
Images were acquired with the Perkin Elmer Operetta HCA and a maximum intensity projection of confocal sections is shown.
-
 Immunocytochemistry/ Immunofluorescence - ioGlutamatergic Neurons – Human iPSC-Derived Glutamatergic Neurons (ab259259)
Immunocytochemistry/ Immunofluorescence - ioGlutamatergic Neurons – Human iPSC-Derived Glutamatergic Neurons (ab259259)Immunofluorescence staining of beta III Tubulin using ab52623 in ioGlutamatergic Neurons (Human iPSC-Derived Glutamatergic Neurons, ab259259), which were differentiated for 11 days post induction.
The cells were fixed with 4% formaldehyde (10 min), permeabilized with 0.1% PBS-Tween for 5 mins and then blocked with 1% BSA/10% normal goat serum/0.3M glycine in 0.1% PBS-Tween for 1h. The cells were then incubated overnight at +4°C with ab52623 at 0.1 μg/ml and ab7291, Mouse monoclonal [DM1A] to alpha Tubulin, at 1/1000 dilution. Cells were then incubated with ab150081, Goat Anti-Rabbit IgG H&L (Alexa Fluor® 488) preadsorbed at 1/1000 dilution (shown in green) and ab150120, Goat Anti-Mouse IgG H&L (Alexa Fluor® 594) preadsorbed at 1/1000 dilution (shown in red). Nuclear DNA was labelled with DAPI (shown in blue).
Images were acquired with the Perkin Elmer Operetta HCA and a maximum intensity projection of confocal sections is shown.
-
 Immunocytochemistry/ Immunofluorescence - ioGlutamatergic Neurons – Human iPSC-Derived Glutamatergic Neurons (ab259259)
Immunocytochemistry/ Immunofluorescence - ioGlutamatergic Neurons – Human iPSC-Derived Glutamatergic Neurons (ab259259)Immunofluorescence staining of MAP2 using ab183830 in ioGlutamatergic Neurons (Human iPSC-Derived Glutamatergic Neurons, ab259259), which were differentiated for 11 days post induction.
The cells were fixed with 4% formaldehyde (10 min), permeabilized with 0.1% PBS-Tween for 5 mins and then blocked with 1% BSA/10% normal goat serum/0.3M glycine in 0.1% PBS-Tween for 1h. The cells were then incubated overnight at +4°C with ab183830 at 1 μg/ml and ab7291, Mouse monoclonal [DM1A] to alpha Tubulin, at 1/1000 dilution. Cells were then incubated with ab150081, Goat Anti-Rabbit IgG H&L (Alexa Fluor® 488) preadsorbed at 1/1000 dilution (shown in green) and ab150120, Goat Anti-Mouse IgG H&L (Alexa Fluor® 594) preadsorbed at 1/1000 dilution (shown in red). Nuclear DNA was labelled with DAPI (shown in blue).
Images were acquired with the Perkin Elmer Operetta HCA and a maximum intensity projection of confocal sections is shown.
-
 Immunocytochemistry/ Immunofluorescence - ioGlutamatergic Neurons – Human iPSC-Derived Glutamatergic Neurons (ab259259)
Immunocytochemistry/ Immunofluorescence - ioGlutamatergic Neurons – Human iPSC-Derived Glutamatergic Neurons (ab259259)Immunofluorescence staining of Tau using ab76128 in ioGlutamatergic Neurons (Human iPSC-Derived Glutamatergic Neurons, ab259259), which were differentiated for 11 days post induction.
The cells were fixed with 4% formaldehyde (10 min), permeabilized with 0.1% PBS-Tween for 5 mins and then blocked with 1% BSA/10% normal goat serum/0.3M glycine in 0.1% PBS-Tween for 1h. The cells were then incubated overnight at +4°C with ab76128 at 0.5 μg/ml and ab7291, Mouse monoclonal [DM1A] to alpha Tubulin, at 1/1000 dilution. Cells were then incubated with ab150081, Goat Anti-Rabbit IgG H&L (Alexa Fluor® 488) preadsorbed at 1/1000 dilution (shown in green) and ab150120, Goat Anti-Mouse IgG H&L (Alexa Fluor® 594) preadsorbed at 1/1000 dilution (shown in red). Nuclear DNA was labelled with DAPI (shown in blue).
Images were acquired with the Perkin Elmer Operetta HCA and a maximum intensity projection of confocal sections is shown.
-
ioGlutamatergic Neurons after revival over the course of the first 11 days of culture. Day 1 to 11 post-thawing; 400X magnification; scale bar: 100µm
-
ioGlutamatergic Neurons display neuronal activity that matures over-time. Examples of MaxOne high-resolution multi electrode array (MEA) recordings of ioGlutamatergic Neurons in BrainPhys™ media. The activity maps show firing rate (A), spike amplitude (B) and % of active electrodes (C). Results demonstrate a time-dependent increase of spontaneous activity during neuronal maturation from 2 to 3 weeks post-revival. Iovino et al., Molecular and functional characterization of stem cells-derived glutamatergic neurons ioGlutamatergic Neurons in support of drug discovery applications., Charles River Laboratories.
-
 High throughput screening - ioGlutamatergic Neurons – Human iPSC-Derived Glutamatergic Neurons (ab259259)
High throughput screening - ioGlutamatergic Neurons – Human iPSC-Derived Glutamatergic Neurons (ab259259)ioGlutamatergic Neurons show good suitability for high-throughput screening in 384-well format plates. Cytotoxicity CellTiter-Glo®️ (CTG) and TR-FRET (HTRF®️) assays for AKT serine/threonine kinase 1 (AKT) and Huntingtin (HTT) proteins were performed on ioGlutamatergic Neurons in 384-well plates treated with tool compound (cmp) at day 9 post-revival. Compound titration results in a concentration response curve for all three assays (mean±sd of 2 replicates). CTG assay on ioGlutamatergic Neurons shows an excellent average signal/ background ratio and high suitability for HTS. HTRF®assays on ioGlutamatergic Neurons show lower signals but with low variability, and could therefore also provide a suitable platform for HTS. Iovino et al., Molecular and functional characterization of stem cells-derived glutamatergic neurons ioGlutamatergic Neurons in support of drug discovery applications., Charles River Laboratories.
-
ioGlutamatergic Neurons are delivered in a cryopreserved format and are programmed to rapidly mature upon revival in the recommended media. The protocol for the generation of these cells is a three-phase process:
1. Induction (carried out at bit.bio)
2. Stabilization for 4 days with Doxycycline
3. Maintenance during which the neurons mature.














